A 55-year-old man is the first person to receive a personalized treatment that gives hope to millions of patients
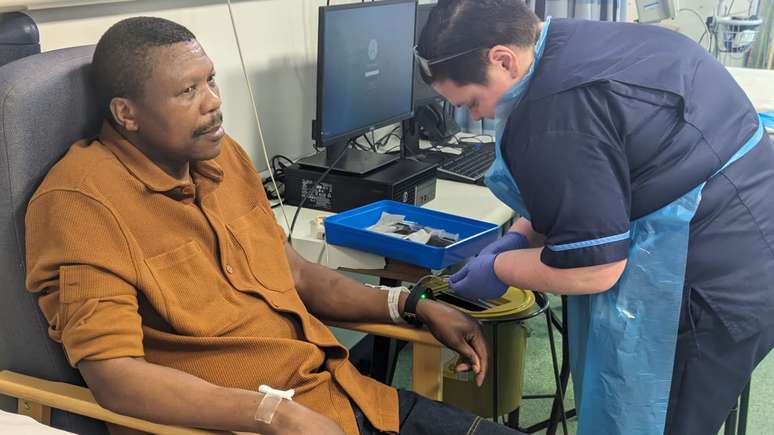
Eliot Febway, a 55-year-old university professor and father of four, became the first patient to receive an experimental personalized vaccine aimed at preventing colon cancer recurrence. The vaccine, developed with mRNA (messenger RNA) technology – the same vaccine used in vaccines against Covid – has the potential to revolutionize oncology treatment.
Friday (31), of the National Health Service UK (NHS) announced the news on Saturday (1st), BioNTech SE, the German biotechnology company responsible for developing the vaccine. A presentation was made during the Annual Medical Oncology Conference in Chicagoto us to us.
Clinical trials aim to evaluate the efficacy and safety of treatments to prevent the recurrence of tumors in patients with severe disease such as Febve. Asymptomatic, he was diagnosed with invasive colon cancer during a routine check-up in 2023. The professor underwent surgery and chemotherapy until the tumor’s symptoms subsided and volunteered for clinical trials. According to a statement, the NHS hopes to recruit more than a thousand people to take part in trials in the coming months.
“If [o estudo clínico] is successful, [a vacina contra o câncer] To be able to help thousands if not millions of people so they can have hope and not go through what I went through”Febway said in a note.
The clinical trial that Febway is part of is one of several funded by the NHS across the country to treat different tumor types. The trials are part of the English National Service’s new oncology vaccine launch platform, which currently brings together more than 30 hospitals across the country. England For patient recruitment and monitoring.
Vaccine development
Vaccines used in colon cancer screening are developed by analyzing a patient’s tumor to identify specific mutations. With this information, doctors develop a personalized vaccine.
These vaccines are designed to induce an immune response that can prevent cancer after surgery on the primary tumor by stimulating the patient’s immune system. At the end of April, another Briton was vaccinated with the first vaccine against skin cancer.
Vaccines are so called because they teach the immune system to fight cancer in the same way that vaccines teach them to protect against viruses and bacteria. However, they are not designed to prevent the development of primary cancer.
Developed jointly by biopharmaceutical companies BioNTech and Genentec, a member of the Roche Group, these vaccines have not yet been approved by regulatory bodies and, therefore, are not available outside of a clinical research context.
* Text under the supervision of Tomas Bellumini